Product Quality Management
Product Quality Management is only valid if it is embodied
PRODUCT QUALITY MANAGEMENT
Product Quality Management is only valid if it is embodied
PRODUCT QUALITY MANAGEMENT
The key challenges of product quality management
Our experience has taught us that these three issues are key to better manage product quality.
75%
reduction of the closing time of ongoings
Removing the Backlog
-20
deviations in progress in the routine portfolio
About
Manage quality to ensure product compliance
• Do you encounter a significant backlog of your deviations?
• Are Root causes not identified and events are recurrent?
• Are deviations not managed and brought under control?
• Is your customer service impacted by delays related to quality issues?
• Do you lack investigative expertise due to high turnover?
A selection of our customers whose quality control has made it possible to guarantee the conformity of the products
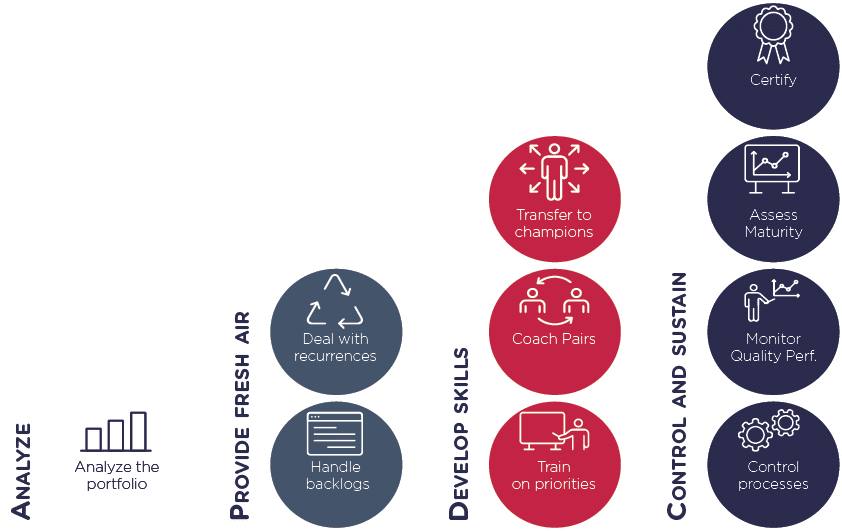
Leverage of actions
Selection

Vous souhaitez que nous étudions votre projet ?Contactez-nous !
BiotechStartupBooster
The innovation project is only valid if it generates value
BIOTECH STARTUP BOOSTER
The innovation project is only valid if it generates value
BIOTECH STARTUP BOOSTER
The key challenges of the Biotech Startup Booster
Two axes are to be considered: the strategic/financial axis and the operational/field axis
Market Access Acceleration
Deployment of an industrial culture
Increase in value
About
Increase value and accelerate growth
• Do you want to co-develop with a commercial, industrial or technological partner?
• Are you looking to strengthen your equity, financial your development program?
• Do you want to secure the scale-up phase and your industrialization?
• Do Your teams have to be trained and integrate industrial reflexes?
• Do you need to install and display regulatory compliance (GMP/GxP/FDA)?
A selection of our customers who asked us to boost their development!
Leverage of actions
Selection

Seeking funds to optimize development costs
Structure and formalize processes to implement a robust Quality system
Mobilize atryon expertise (PMO, managers, etc.) on key functions to accelerate processes (QA/QC, development, manufacturing, supply chain)
Align capacities, means of production and processes with the development roadmap
Train and coach teams in GxP/Aseptic Culture
Vous souhaitez que nous étudions votre projet ?Contactez-nous !
Enhanced eBR
Digitalization is only valid if it is based on relevant and robust data
ENHANCED eBR
Digitalization is only valid if it is based on relevant and robust data
ENHANCED eBR
Data Analytics & Big Data for Industry 4.0
The key challenges of digitization of the Batch Records
Our experience has taught us that these three issues are key to the digitization of your Batch Records.
50%
streamlined and simplified data
10%
reduction of material costs
80%
reduction of cycle time
by the way
Digitize your batch file while optimizing your industrial performance
• Are you having trouble keeping up to date too many master data ?
• Are you finding it difficult to consolidate your data to fuel Continuous Improvement?
• Do you have significant costs related to the file review and release processes?
• Your cycle time does not allow you to meet your customer commitments?
Leverage of actions
Selection

Streamline and Simplify Batch Folders
• Identify critical parameters
• Analyze data criticalities
• Adhere to flows
Monitor the process(es) in real time
• Implement eBR / Paper-on-glass
• Measuring OEE on lines
• Install control cards on lines
Optimize the process(es)
• Deploy Analytics
• Make Data vizualization on your equipment
• Get automated reportings











